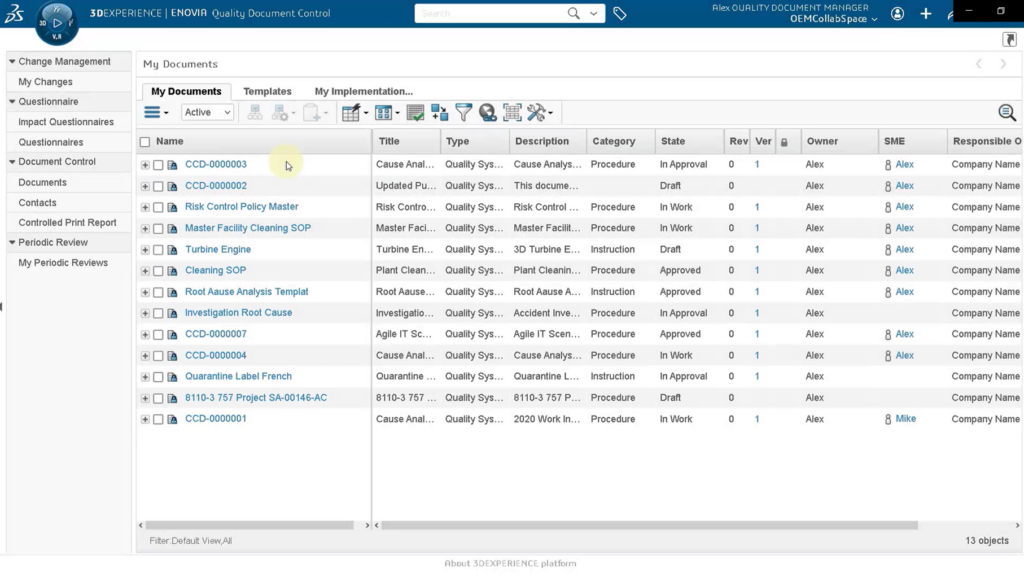
Does your organization need to store and manage quality records? How does your company ensure that quality documents undergo a controlled release process and are updated in a timely manner? Is your organization looking for a way to track and manage who has been trained on new and revised documents? Dassault Systèmes’ ENOVIA Quality Document Author app on the 3DEXPERIENCE platform is a collaborative, enterprise-wide Document Control solution that can help.
3DEXPERIENCE ENOVIA Quality Document Author
The Quality Document Author app manages authoring, reviewing, releasing and publishing of controlled quality documents, while ensuring regulatory compliance. Quality Document Author uses a common change process leveraging change impact analysis, where used functionality across all domains, while maintaining a traceable reliable audit trial of changes and read and understood statements.
Product Quality Document Single Source of Truth
Document control is the process of applying policies and rules to how documents are created, stored, updated and retired. Any product document that needs to be controlled for quality management is treated as a Quality Document in 3DEXPERIENCE ENOVIA. These could include Standard Operating Procedures, Work Instructions, Purchase Orders, Specifications, Drawings, etc. Quality Document Author enforces document control management using a standard global change control process across all organizations to help enforce document control compliance requirements and supports the following regulations:
- 21 CFR 820 Quality System Regulation (GMP — Good Manufacturing Process)
- 21 CFR 11 (ERES — Electronic Records and Electronic Signatures)
- ISO 13485 (Clause 4) for establishment of a Quality management system
- ISO 9001 Quality Standard
Standardize Controlled Documents
Users of Quality Document Author can standardize document control by creating templates for each document type. Template creation is the start of an electronic workflow of tasks and controls assignments, alerts, escalations, and reviews that can be automated to the level you prefer for enterprise document change and training management.
The templates contain a standardize file that is used as basis for quality documents created from that template. Quality Document Authors can also configure attribute groups, responsible Quality SME and storage classification by Document Type.
The system automatically maintains a fully compliant, detailed electronic archive of all templates. It also aligns stakeholders and communicates changes with specific sequencing of the approval tasks.
Approve & Release Documents
Quality Document Author controls changes by identifying ownership of the change, allows for review and approval of the changes by stakeholders (supports CFR 21 Part 11) preventing changes that could adversely affect product quality or conflict with requirements.
Change managers are able to define standard impact questionnaires to perform where use analysis and impact assessments to identify potentially related affected items and training requirements. Questionnaires are used to for configuring required approvers along with support for escalations reminders for overdue task approvers.
During the change process, approvers can review the data undergoing the change, provide justification/feedback, and perform relevant markups to capture their review comments. The history of changes and comments are captured and stored in the 3DEXPERIENCE platform which also supports audit compliancy purposes.
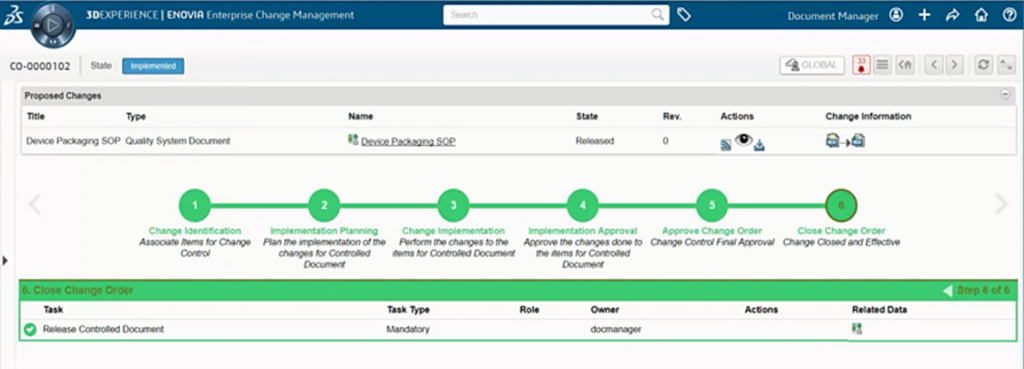
These change processes can be used to update, release, or mark the quality document as obsolete. The 3DEXPERIENCE platform ensures all concerned locations and users are aware that documents have undergone changes by notifying them automatically, which is an important feature of being quality compliant.
Track User Training
When quality documents are revised or released, all concerned users should to be trained on these changes. Quality Document Author provides a simple way to schedule and record training for the people in the organization. Users can easily view their responsible documents, templates and periodic reviews that require their attention. Users can leverage document filtering to access released documents, responsible organization, subject matter expert, implementation organization, and responsible person. Training can be assigned to a group of or individual users.
Users get an automatic notification of upcoming or pending training and must electronically sign-off once the training is complete which is mandatory for regulatory compliance.
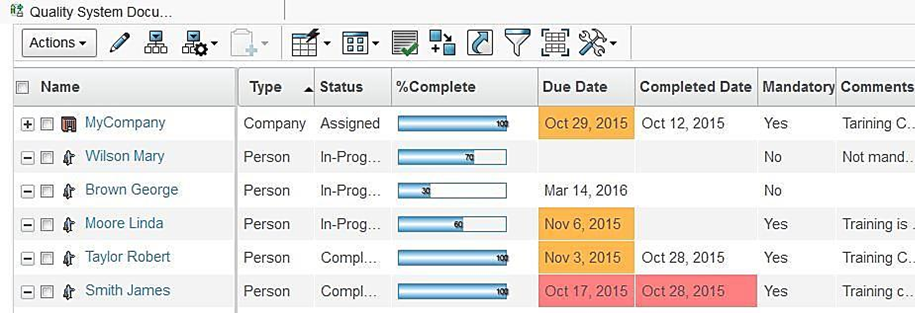
Implementation managers can track training progress of participants for quality documents they are in charge off. In addition to percent completion, 3DEXPERIENCE ENOVIA will also color code the training due dates of participants so that managers can ensure on-time completion of training.
Periodic Review
Quality Documents are automatically setup for periodic reviews based on effectivity dates. Documents are sorted by review dates so quality managers can identify which control documents have to be reviewed and updated immediately. Quality Managers must sign-off on the completeness of the document or pass it through a change process for further modifications. This allows a complete 360-degree quality review and implementation of current best practices.
Watermark Control Information
3DEXPERIENCE ENOVIA will generate PDF files for distribution with configuration setting by document types that includes information setting about document lifecycle, headers, footers, watermarks and change approvers.
User can manage distribution and tracking of hard copy documents with a unique serial number including the ability to recall printed hardcopy documents. This ensures users know they are dealing with a Controlled Document when it is printed out or viewed in the system. A batch process is triggered whenever the document is modified and ensures the PDF document has up-to-date watermarks when viewed.
Summary
In summary, 3DEXPERIENCE ENOVIA Quality Document Author drives a centralized standard process to manage and release quality documents while ensuring that all concerned parties are trained on new and revised documents. This supports product quality improvements, sets up a foundation for GMP (Good Manufacturing Process), and provides a basis for companies to be CFR and ISO compliant.
Other Resources
View this webinar to understand Dassault Systèmes’ broader Quality solutions portfolio: From Reactive to Proactive Product Quality Manager
Questions?
If you have any questions or would like to learn more about the ENOVIA Quality Document Author app on the 3DEXPERIENCE platform, please contact us at (954) 442-5400 or submit an online inquiry.