Any document that needs to be controlled for quality management is treated as a Quality Document in ENOVIA. These could include Standard Operating Procedures, Work Instructions, POs, Specifications, Drawings, etc. Let’s explore how ENOVIA manages these controlled documents and can help your company maintain ISO 9001 compliance.
Prevent Use of Obsolete Documents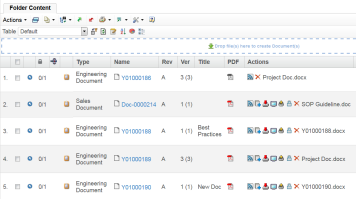
ENOVIA Quality Document Manager provides a single global reference for quality documents. These documents are revision and access controlled based on project assignment.
Downstream users always access the latest released revision of the document but can also access document history to compare details of earlier versions. This ensures that users only work with the latest and greatest document revision.
Quality managers can also create a Quality Document Template for different types of quality documents to capture best practices and enforce company-wide standards.
Approve & Release Documents
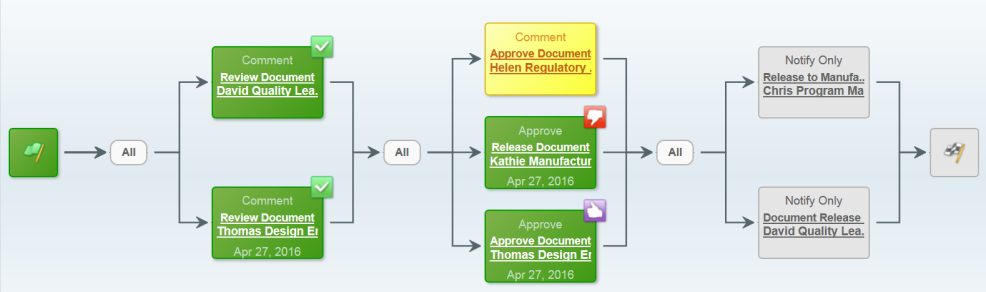
ENOVIA Quality Documents effectively manages the approval and release of controlled documents using a robust change process. ENOVIA provides change managers with the unique ability of selecting appropriate approvers based on the type of quality document undergoing the change process.
During the change process, approvers can review the data undergoing the change, provide justification/feedback and perform relevant markups to capture their review comments. The history of changes and comments are captured and stored in ENOVIA for audit compliancy purposes as well.
These change processes can be used to update, release or mark the quality document as obsolete. ENOVIA ensures all concerned locations and users are aware that documents have undergone changes by notifying them automatically, which is an important feature of being quality compliant.
Track User Training
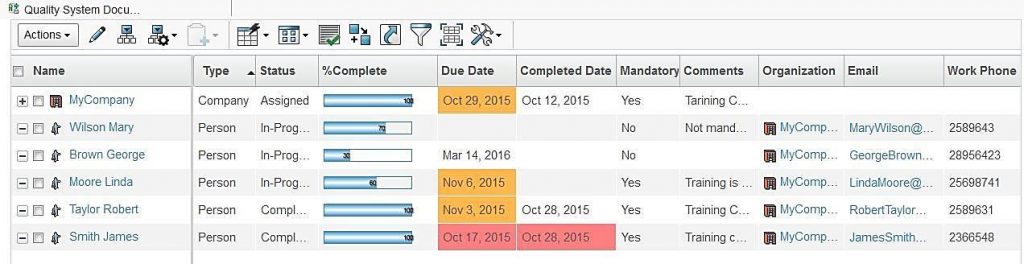
Once documents are revised and released, all concerned users have to be trained on these changes. ENOVIA Quality Document Management tracks assignment of training to users and their progress. Training can be assigned to a group of or individual users.
Users get an automatic notification of upcoming or pending training and have to electronically sign-off once the training is complete which is mandatory for regulatory compliance.
Implementation managers can track training progress of participants for quality documents they are in charge off. In addition to percent completion, ENOVIA will also color code the training due dates of participants so that managers can ensure on-time completion of training.
Periodic Review
Quality Documents are automatically setup for periodic reviews based on effectivity dates. Documents are sorted by review dates so quality managers can identify which control documents have to be reviewed and updated immediately. Quality Managers have to sign-off on the completeness of the document or pass it through a change process for further modifications. This allows a complete 360 degree quality review and implementation of current best practices.
Watermark Control Information
ENOVIA can save Quality Documents as a PDF and watermark the file with control information such as current document state (Draft, Release), timestamp, owner, document attributes, etc. This ensures users know they are dealing with a Controlled Document when it is printed out or viewed in the system. A batch process is triggered whenever the document is modified and ensures the PDF document has up-to-date watermarks when viewed.
In Summary, ENOVIA Quality Document Manager drives a centralized standard process to manage and release quality documents while ensuring that all concerned parties are trained on new and revised documents. This sets up a foundation for GMP (Good Manufacturing Process) and provides a basis for companies to be CFR and ISO compliant.
For more information, reach out to us at info@inceptra.com
Article Submitted By
Anita Mathias