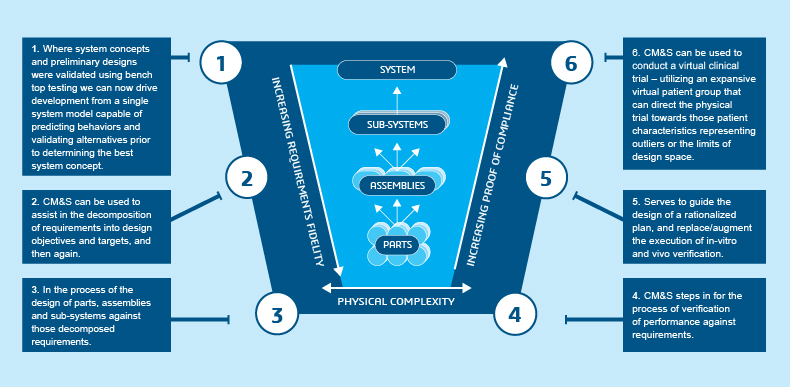
The Role of Computational Modeling & Simulation (CM&S) in Product Development
The medical device industry today faces rising pressure from healthcare communities, patients wanting more personalized care, consumers, and policy makers to lower healthcare costs, reduce time and costs to market for new products, and comply with increasing onerous regulations. Reducing time and cost to market while maintaining quality and patient safety. Addressing these points is crucial for the financial stability, growth, and innovative capabilities of the company.
This has prompted organizations to re-examine their processes, including, pre-clinical verification and validation (V&V) processes. The design and V&V processes heavily rely on bench testing of physical prototypes, followed by animal studies and human clinical trials. However, this process has two significant limitations:
- There are interactions and effects between device and patient that simply cannot be adequately tested nor observed at the bench
- It is challenging to adequately quantify risk and test for safety with animal studies and human clinical trials
Therefore, the medical device industry is evolving towards a model that leverages Computational Modeling & Simulation (CM&S) to lessen the burden of physical trials, build the products that will improve both the patient experience and patient outcomes, and better understand and qualify the risks.
Complete the form to read our white paper.
The Expanding Role of CM&S in Medical Device MBSE
Limited availability of closed form numerical solutions restricts the ability of design engineers in the medical device industry to develop optimal product functionality. Consequently, physical testing has traditionally dominated this industry. However, the integration of CM&S into the Model-Based Systems Engineering (MBSE) paradigm is revolutionizing the field of medical device development.
Discover More
CORPORATE REPORTS
Discover the Latest Innovative Projects, Including:
The Living Heart Project
With the support of Dassault Systèmes 3DEXPERIENCE® platform, companies can design the most effective and least invasive devices. At the same time, ensuring that care pathways reflect their patients’ full diversity and personalize care affordably.

CORPORATE REPORTS
A New Leader in Life Sciences
The virtual twin of the human body created on Dassault Systèmes 3DEXPERIENCE platform will help cure people and help them lead a better life.